How Much Water Do You Need To Add To 500 Mls Of 2.4m Kcl Solution To Make A 1m Solution?
ane.8: Serial Dilutions and Standard Curve
- Page ID
- 36750
Learning Objectives
Goals:
- Prepare solutions starting with a solid.
- Perform a series dilution.
- Apply the spectrophotometer to measure the absorbance of solutions.
- Generate a standard curve and use the standard bend to make up one's mind the concentration of a solution.
Educatee Learning Outcomes:
Upon completion of this lab, students will be able to:
- Determine the mass of solute needed to brand at %(west/v) solution.
- Make a buffer of the appropriate concentration.
- Brand a stock solution of the appropriate concentration.
- Create a series of solutions of decreasing concentrations via serial dilutions.
- Use the spectrophotometer to measure the absorbance of a solution.
- Apply excel and brand a standard curve and use the R2 value to evaluate the quality of the standard curve.
- Use the standard bend to calculate the concentration of a solution.
Introduction
A Serial dilution is a serial of dilutions, with the dilution factor staying the same for each step. The concentration factor is the initial volume divided past the final solution volume. The dilution factor is the inverse of the concentration cistron. For instance, if you take one part of a sample and add 9 parts of water (solvent), and then you have made a 1:x dilution; this has a concentration of 1/10th (0.1) of the original and a dilution factor of 10. These dilutions are oftentimes used to determine the approximate concentration of an enzyme (or molecule) to be quantified in an analysis. Series dilutions allow for small aliquots to be diluted instead of wasting large quantities of materials, are toll-effective, and are piece of cake to gear up.
\[concentration factor= \frac{volume_{initial}}{volume_{concluding}}\nonumber\]
\[dilution factor= \frac{one}{concentration cistron}\nonumber\]
*Dilution tubes begin with 9-mL. 1-mL is added and mixed, then 1-mL is transferred to the next tube. The catastrophe volume in the concluding tube would exist 10-mL
Key considerations when making solutions:
- Make sure to always research the precautions to use when working with specific chemicals.
- Be certain yous are using the right form of the chemical for the calculations. Some chemicals come as hydrates, meaning that those compounds contain chemically bound water. Others come as "anhydrous" which means that there is no bound h2o. Be sure to pay attending to which i you are using. For example, anhydrous CaClii has a MW of 111.0 grand, while the dehydrate course, CaCltwo ● 2 H2O has a MW of 147.0 grams (110.0 one thousand + the weight of ii waters, 18.0 grams each).
- Always employ a graduate cylinder to mensurate out the amount of h2o for a solution, utilise the smallest size of graduated cylinder that will accommodate the unabridged solution. For example, if y'all need to brand 50 mL of a solution, it is preferable to utilize a l mL graduate cylinder, merely a 100 mL cylinder can be used if necessary.
- If using a magnetic stir bar, be sure that it is clean. Do not handle the magnetic stir bar with your bare hands. Y'all may desire to wash the stir bar with dishwashing detergent, followed by a consummate rinse in deionized h2o to ensure that the stir bar is clean.
- For a 500 mL solution, start by dissolving the solids in about 400 mL deionized h2o (normally near 75% of the terminal volume) in a chalice that has a magnetic stir bar. And then transfer the solution to a 500 mL graduated cylinder and bring the volume to 500 mL
- The term "bring to volume" (btv) or "quantity sufficient" (qs) means adding water to a solution you are preparing until it reaches the desired total book
- If you demand to pH the solution, do so BEFORE you bring upwardly the volume to the terminal volume. If the pH of the solution is lower than the desired pH, then a stiff base (often NaOH) is added to heighten the pH. If the pH is higher up the desired pH, and then a strong acrid (often HCl) is added to lower the pH. If your pH is very far from the desired pH, apply higher molarity acids or base. Conversely, if you are close to the desired pH, use depression molarity acids or bases (like 0.5M HCl). A demonstration volition be shown in grade for how to use and calibrate the pH meter.
- Characterization the bottle with the solution with the following information:
- Your initials
- The name of the solution (include concentrations)
- The date of grooming
- Storage temperature (if you lot know)
- Label hazards (if there are whatsoever)
Lab Math: Making Percentage Solutions
Formula for weight pct (due west/v):
\[ \dfrac{\text{Mass of solute (g)}}{\text{Volume of solution (mL)}} \times 100 \nonumber \]
Make 500 mL of a 5% (w/five) sucrose solution, given dry out sucrose.
- Write a fraction for the concentration \[5\:\%\: ( \frac{west}{v} )\: =\: \dfrac{5\: g\: sucrose}{100\: mL\: solution} \nonumber\]
- Set up a proportion \[\dfrac{5\: m\: sucrose}{100\: mL\: solution} \:=\: \dfrac{?\: one thousand\: sucrose}{500\: mL\: solution} \nonumber\]
- Solve for thousand sucrose \[\dfrac{v\: g\: sucrose}{100\: mL\: solution} \: \times \: 500 \: mL \: solution \: = \: 25 \: grand \: sucrose \nonumber\]
- Add 25-g dry NaCl into a 500 ml graduated cylinder with enough DI water to dissolve the NaCl, then transfer to a graduated cylinder and fill up to 500 mL total solution.
Activity 1: Calculating the Amount of Solute and Solvent
Calculate the corporeality (include units) of solute and solvent needed to make each solution.
A. Solutions with Soluble Solute and h2o as the solvent
- How many grams of dry out NaCl should be used to make 100 mL of fifteen% (W/5) NaCl solution?
- How many grams of dry NaCl should be used to make 300 mL of 6% (W/V) NaCl solution?
- How many grams of dry NaCl should exist used to make 2L of 12% (W/V) NaCl solution?
- How many grams of dry NaCl should be used to make 300 mL of 25% (West/5) NaCl solution?
- How many grams of dry out NaCl should be used to make 250 mL of 14% (W/Five) NaCl solution?
B. Solutions with Insoluble Solutes in Cold H2o
- Calculate how to prepare 200 mL ane.two% (w/v) agarose in 1X SB buffer, given dry agarose and SB buffer.
- Calculate how to ready 300 mL 2.5 % (due west/v) agarose in 1X SB buffer, given dry out agarose and SB buffer.
- Calculate how to fix l mL 1.5 % (w/v) agarose in 1X SB buffer, given dry agarose and SB buffer.
- Calculate how to prepare sixty mL 0.8 % (w/5) agarose in 1X SB buffer, given dry agarose and SB buffer.
- Summate how to fix 150 mL 1.eight % (west/v) agarose in 1X SB buffer, given dry out agarose and SB buffer.
For dry chemicals that cannot dissolve in cold h2o (such as agarose and gelatin), pour the dry solute directly into an Erlenmeyer flask, measure out the total volume of solvent in a graduated cylinder, then add the total volume of solvent into flask. Microwave the solution as recommended until solute is dissolved.
Role I: Solution Prep of 30-mLs of 13.vi% Sodium Acetate
Sodium Acetate Buffer solutions are inexpensive and ideal to practice your skills. Your accuracy can be verified by taking a pH reading .
MATERIALS
Reagents
- Sodium Acetate (Trihydrate) solid
- DI H2O
- Stock bottle of verified 1 Tooth Acetic Acrid solution
Equipment
- pH meter
- Stir plate
- Electronic balance and counterbalance boats
- 50-mL graduated cylinder
- l-mL conical tubes (Falcon tubes)
- P-k Micropipettes with disposable tips (or 5 mL Serological pipettes with pumps)
Calculations
- Calculate the amount of sodium acetate needed to make 30 mL of 13.vi% sodium acetate solution.
Procedure
- Make sure to clothing goggles and gloves.
- Measure _______ 1000 of solid sodium acetate in a weigh boat on an electronic residuum.
- Transfer the sodium acetate into a 50 mL conical tube.
- Add about 20 mL of DI water into the conical tube.
- Secure the cap on the tube and capsize to mix the contents until the solute is completely dissolved.
- Cascade out all of the solution into a l mL graduated cylinder.
- Add DI water to bring the total volume to 30.0 mL.
- Transfer all of the solution back into your l mL conical tube and secure the cap.
- Capsize the tube several times to thoroughly mix the contents.
- Label the tube with contents (13.6% Sodium Acetate), initial, and date.
Verify your work past creating a buffer solution
- Pipette exactly v.0 mL of your sodium acetate solution into a clean xv mL conical tube (or 25 mL glass test tube).
- Pipette exactly v.0 mL of 1M acetic acid solution into your conical tube (or 25 mL glass test tube).
- Secure the cap on the conical tube (or a piece of parafilm over the test tube opening).
- Invert several times to thoroughly mix the 10 mL of solution into an acetate buffer.
- Measure the pH of the exam buffer solution using a calibrated pH meter.
- If y'all were accurate in all of your work, the examination buffer should have a pH of iv.75 (+/- 0.06).
- Check in with your teacher and report the pH of your test buffer.
- If your test buffer pH is within the expected range, then congratulations! You have verified that the sodium acetate solution you made earlier has a concentration of thirteen.six%. Give your l mL tube of remaining sodium acetate solution to your instructor to salve for use in a future lab.
- If your test buffer pH is far outside of the expected range and then something went wrong during the preparation of your sodium acetate solution and you should mark the tube with an "X" and give it your teacher to ready aside.
Role II: Training of a Standard Curve
In this part of the lab, nosotros will be preparing solutions of known concentrations. These then will be used to create a standard curve. Standard curves (also known as calibration curves) represent the relationship between two quantities. The standard bend will exist used in part 3 of the lab to determine the concentrations of unknown solutions of methylene bluish.
Materials
Reagents
- Stock i% (w/v) methylene bluish solution – (500 microliter (µL) aliquots in one.5 mL microcentrifuge tubes)
- DI H2O
Equipment
- P-twenty Micropipettes and disposable tips
- P-1000 Micropipettes and disposable tips
- Spectrophotometer
Glassware
- 10 mL serological pipettes and pumps
- 1.v mL microcentrifuge tubes
- 15 mL plastic conical tubes with screw-top caps
- 50 mL plastic conical tubes with screw-meridian caps
Calculations
- Calculate the volume of stock 1% methylene blue solution needed to make 40 mL of 0.0005 % methylene blue solution.
- This new percent concentration is equivalent to 5.0 micrograms per milliliter (µg/mL) and will be the concentration of our working solution for the next ii parts of the lab exercise.
Procedure
Prepare Stock Solution of Methylene Blue
Prepare 40 mL of 5.0 µg/mL Methylene Blue Working Solution
- Make sure to vesture goggles and gloves.
- Very accurately pipette forty.0 mL of DI water into a 50 mL conical tube.
- Very accurately micropipette ________ µL of ane% stock methylene bluish into the DI water in your tube.
- Secure the cap on the tube and invert repeatedly to thoroughly mix the solution.
- Label your tube every bit "5.0 µg/mL Methylene Blue", your name, and date.
Prepare Known Concentrations of Methylene Blue Working Solution via Dilution
Ready 80% Methylene Bluish Working Solution
- Pipette viii.0 mL of 5.0 µg/mL methylene blueish working solution into a 15 mL conical tube.
- Pipette two.0 mL DI Water into the tube to make 10.0 mL of total solution.
- Seal the tube and invert repeatedly to mix.
- What is the concentration of your new solution? Label the tube _______ µg/mL methylene blue.
Set 60% Methylene Blue Working Solution
- Pipette half dozen.0 mL of 5.0 µg/mL methylene blue working solution into a 15 mL conical tube.
- Pipette iv.0 mL DI H2o into the tube to brand x.0 mL of total solution.
- Seal the tube and capsize repeatedly to mix.
- What is the concentration of your new solution? Label the tube _______ µg/mL methylene blue.
Prepare forty% Methylene Blueish Working Solution
- Pipette 4.0 mL of 5.0 µg/mL methylene blue working solution into a fifteen mL conical tube.
- Pipette 6.0 mL DI H2O into the tube to make x.0 mL of total solution.
- Seal the tube and capsize repeatedly to mix.
- What is the concentration of your new solution? Label the tube _______ µg/mL methylene blue.
Ready 20% Methylene Blueish Working Solution
- Pipette 2.0 mL of 5.0 µg/mL methylene blueish working solution into a 15 mL conical tube.
- Pipette 8.0 mL DI H2O into the tube to make 10.0 mL of full solution.
- Seal the tube and invert repeatedly to mix.
- What is the concentration of your new solution? Label the tube _______ µg/mL methylene blue.
Measuring Absorbance of Methylene Blue Working Solutions
- Turn on the spectrophotometer and allow it warm upwardly for at least 10 minutes.
- Place i mL of DI water into a clean cuvette. This is your blank.
- Place 1 mL of your methylene bluish solutions into clean cuvettes. These are your samples.
- Prepare the wavelength of the spectrophotometer to 664 nm.
- Identify the blank into the spectrophotometer.
- Press the "Zero" push button and wait for the Absorbance to read "0.00"
- Accept out the blank and fix aside.
- Place your outset sample into spec and record the absorbance reading. Practise not press whatever buttons.
- Repeat with each sample and record into lab notebook
Results
Complete Data Table 1. based on your results. Put in your notebook
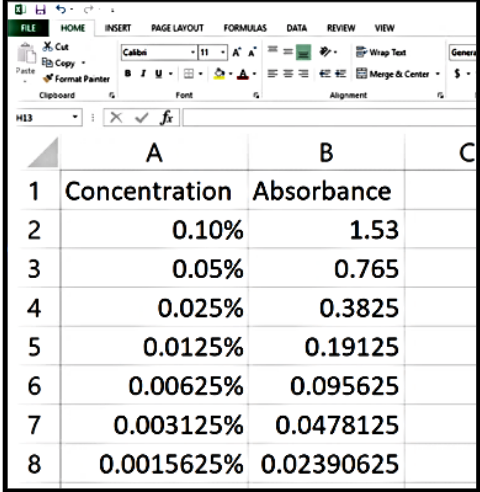
| Pct of Working Solution Conc. | Methylene Blue Concentration (µg/mL) | Absorbance @ 664 nm |
|---|---|---|
| 100% | 5.0 | |
| 80% | ||
| 60% | ||
| 40% | ||
| xx% |
Making a Standard Curve
- Enter the data into Excel in adjacent columns.
- Select the data values with your mouse. On the Insert tab, click on the Scatter icon and select Besprinkle with Straight Lines and Markers from its drop-down menu to generate the standard curve.
- To add a trendline to the graph, right-click on the standard curve line in the nautical chart to brandish a pop-upwards carte du jour of plot-related actions. Choose Add Trendline from this menu. Select "display equation on chart" and "display R-squared value on nautical chart". Ideally, the R2 value should be greater than 0.99.
- Use the equation to determine the concentration of the sample solution by entering the absorbance for y and solving for x.
- Impress the standard curve and add together to your notebook.
Role Iii: Determining Concentrations
Serial dilutions are quick manner of making a set of solutions of decreasing concentrations. In this part of the lab we volition make a series of dilutions starting with the Methylene Blue solution prepared in part 2 of this lab. Then, we will us the spectrophotometer to make up one's mind the absorbance of each solution. In one case we know the absorbance, we will use the equation from your standard curve prepared in role 2, to make up one's mind the bodily concentrations of each of your solutions.
Materials
Reagents
- 5.0 µg/mL Methylene Blue Working Solution
- DI Water
Equipment
- P-twenty Micropipettes and dispensable tips
- P-k Micropipettes and dispensable tips
- Spectrophotometer
- 5 mL serological pipettes and pumps
- xv mL plastic conical tubes with screw-top caps
Grooming of Methylene Blueish Solutions
Using the remainder of your 5.0 µg/mL methylene blue working solution from part 2, perform a set of ane:ii serial dilutions to make the following concentrations of the solution (50.0 %, 25.0 %, 12.5 %, 6.25 %, 3.125 %, and 1.5625 %).
Diagram of 1:2 Serial Dilutions
In your notebook, draw a diagram showing the serial dilutions for the vi methylene blue solutions you are preparing. In the diagram, indicate the volume beingness withdrawn from the full-bodied solution, the book of water added, the concentration of the new solution, and the full volume.
Process
Preparation of Methylene Blue Concentrations via Serial Dilutions
Making 1:2 dilutions
- Pipette 5.0 mL of the five.0 µg/mL methylene blue working solution into a xv mL conical tube.
- Pipette v.0 mL of DI water into the tube for a total of x mL of solution.
- Cap and mix well.
- Label this tube "50.0% MB"
Making 1:iv dilution
- Pipette 5.0 mL of the l.0% MB solution into a new 15 mL conical tube.
- Pipette 5.0 mL of DI water into the tube for a total of ten mL of solution.
- Cap and mix well.
- Label this tube "25.0% MB"
Making i:eight dilution
- Pipette five.0 mL of the 25.0% MB solution into a new fifteen mL conical tube.
- Pipette v.0 mL of DI water into the tube for a total of 10 mL of solution.
- Cap and mix well.
- Label this tube "12.5% MB"
Go along with this process to brand the one:16, 1:32, and ane:64 series dilutions.
Write the procedures you used to make the solutions in your lab notebook.
Measuring absorbance
- Follow the procedures in function 2 to fix the spectrophotometer
- Measure the absorbance values of the diluted solutions
- Record the absorbance values and concentrations in your lab notebook in a table equally shown below.
| Dilution Factor | % of Working Solution Concentration | Absorbance @ 664 nm | Methylene Blue Conc. (µg/mL) |
|---|---|---|---|
| 1:2 | 50.0% | ||
| 1:4 | 25.0% | ||
| one:eight | 12.five% | ||
| one:16 | 6.25% | ||
| 1:32 | 3.125% | ||
| 1:64 | 1.5625 % |
Calculations
Use the equation from your standard curve in office 2 and the absorbance values of your solutions from Role three, to make up one's mind the actual concentration of your solutions.
Study Questions
- Describe how you would set up 50.0-mL a 0.10% NaOH solution. In your description, include a calculation and pace by step procedures including glassware.
- It is common for solutions that are used often in a lab (or which are time consuming to ready) to be intentionally prepared to exist many times more than concentrated than needed. For instance, if a 1.36% sodium acetate is often used in the lab, then the 13.vi% sodium acetate solution prepared in function 1 can exist labeled as "10X" sodium acetate solution because the concentration is 10 times greater than needed. This mode, you tin can relieve on storage space for the solution and you lot can speedily and hands dilute whatsoever desired amount of this to the correct concentration right before use.
- Describe how you would prepare 100.0 mL of 10X sodium acetate solution. In your description, include a calculation and step by pace procedures including glassware. Make sure to include steps to verify your solution past checking the pH.
- Describe how you lot would prepare 100.0 mL of 1X sodium acetate solution from the 10x sodium acetate solution prepared in the questions above. In your description, include a calculation and step by step procedures including glassware.
- Using a serial dilution, describe how you lot would prepare ten mL of a i%, 0.i% and 0.01% solution of NaOH. The stock solution of NaOH is 10%. Draw diagram as part of your description.
- Using the standard curve beneath, summate the concentration of an unknown solution if its absorbance is 0.55.
Effigy 3. A standard absorbance bend of Copper II - Evaluate the quality of the standard curve above past using the R2 value.
How Much Water Do You Need To Add To 500 Mls Of 2.4m Kcl Solution To Make A 1m Solution?,
Source: https://bio.libretexts.org/Bookshelves/Biotechnology/Lab_Manual%3A_Introduction_to_Biotechnology/01%3A_Techniques/1.08%3A_Serial_Dilutions_and_Standard_Curve
Posted by: parkerthavercuris.blogspot.com


0 Response to "How Much Water Do You Need To Add To 500 Mls Of 2.4m Kcl Solution To Make A 1m Solution?"
Post a Comment